Draw the Lewis Structure for Sih4
Find out what lewis structure for sih4 means, learn different structures of covalent compounds and see how they are represented in the form of Lewis formula. This article Show What is lewis structure for sih4? Learn how to draw the lewis structure for sih4. This article teaches you all the basics about lewis structures, including important tips and tricks on drawing them.
What is lewis structure for sih4?
Chemists use Lewis structures to represent molecules by showing the valence electrons as dots around the atoms. The sih4 molecule has 8 valence electrons, 4 on each of its two central boron atoms and 2 on each of its four peripheral hydrogen atoms.
Lewis structures are a way to show the structure of molecules that have more than one atom. They were first developed by Gilbert Lewis in 1916 and can be used to determine how electrons are shared between atoms. This is useful because it helps us understand the reactivity of a molecule, which is often related to its shape or geometry. Sih4 has two chlorine atoms bonded together with four hydrogen atoms around them as shown below:
A lewis dot diagram for sih4 looks like this:
This shows that both chlorine atoms share their electron pairs equally with each other, but they don't share any electrons with the hydrogen ions on either side of them. So these two Cl-Cl bonds are equivalent and should behave identically.
Is lewis structure for sih4 polar or nonpolar?
Lewis structures are useful for determining whether an atom is polar or nonpolar. Sih4 has a total of 12 valence electrons (12e). 8 of these electrons will be in the outermost electron shell while 4 will be in the second outermost shell. The sulfur atom, which is central to this molecule, has 6 valence electrons and 2 lone pairs that exist outside its octet. This means that the sih4 molecule is polar with partially filled p-orbitals on both sides of the plane of symmetry.

In truth, both answers are correct. The reason that there can be two answers for one specific molecule is that the electron density around different atoms within a molecule can change its polarity. This means that even if an atom has not changed position, such as with SiH4 (silane), it could still have a polar or nonpolar reaction depending on where it is located in relation to other atoms within the molecule. However, when looking at molecules overall we usually use geometric criteria like shape and bonding angles.
Lewis Structure SiH4 Molecular Geometry
Lewis Structures are a type of structural representation that show the spatial arrangement of all valence electrons and the molecular geometry. The atoms in Lewis structures are represented by circles, with lines or arrows to indicate bonds between them.
If there is more than one bond between two atoms, then there should be a line for each bond. In the case of SiH4, if we were to draw it as a Lewis structure, we would have four hydrogen atoms covalently bonded to one silicon atom with an octahedral symmetry. This means it has six equivalent equilateral triangles forming its corners where three points meet at each vertex (see image). It also has four different types of angles: 120° ∠AOB; 90°.
Many elements in the periodic table have been studied extensively, but there are some that have not been researched enough. One of these is silicon hydride SiH4. There has been a lot of research on other molecular geometries for this element such as trigonal bipyramidal and octahedral, but little research has focused on the tetrahedral geometry.
How do you draw the Lewis structure for SiH4?
The Lewis structure is a way to show how many valence electrons an atom has. This diagram shows the number of pairs of shared electrons around each atom in the compound, which is SiH4. The shared pairs are represented by dashes on lines that connect two different atoms together.
Each hydrogen atom only needs one electron for its single bond to form with another atom, but the silicon needs four atoms to complete its octet and have eight total electrons in order to be stable. You can see this since there are four dashes surrounding it because it shares four pairs of electrons with other atoms.
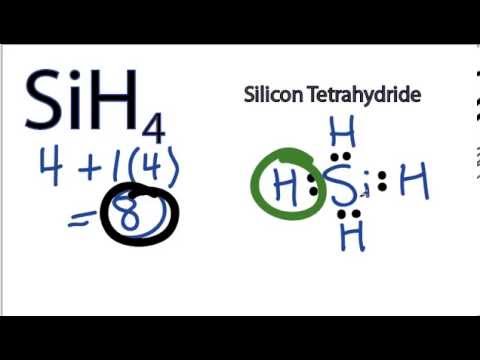
The Lewis structure for SiH4 is a bit different than most molecules. It's not all that common, but it does pop up in the lab every once in a while. This short post will show you how to draw the Lewis structure of SiH4 and why it's important.
What is the angle of SiH4?
The angle of SiH4 is defined as the acute or obtuse angle formed by two lines, one on each side of a planar molecule. The bond angles are around 109 degrees and the bond length is about 1.63 angstroms. This compound can also be called silane, which has four hydrogen atoms bonded to a silicon atom that forms an octahedral geometry or shape.
The angle of SiH4 is the most difficult to measure, as it has a very small energy barrier. This means that there is not enough separation between the different states of energy for it to be easily measured. The chemical bond angle in SiH4 is 109° and this cannot be changed by any other force or compound (most commonly another molecule), so we know the value must stay at 109° because anything else would mean an unstable molecule.
Is SiH4 square planar?
The article discusses whether SiH4 is square planar. It also touches on the different forms of symmetry and how they apply to this molecule's shape. The author notes that there are many inaccuracies in the way this molecule has been shown as a square planar, but it does not mean that it cannot exist as such under certain conditions.
The authors note that because chemists typically draw molecules using two-dimensional representations, these can be misleading because we do not see all three dimensions at once. They conclude by saying that because of this, chemists should avoid drawing molecules with four or more bonds and instead use models where they could show multiple views simultaneously (i.e., 3D).
Read More:
- How many cups in 24 oz?
- How to convert 81cm to inches?
- How to convert 60 mm in inches?
- Who is Aj Hawk wife?
- Are jellies Osrs worth killing?
staufferinece1988.blogspot.com
Source: https://phootoscelebrities.com/what-is-lewis-structure-for-sih4/
0 Response to "Draw the Lewis Structure for Sih4"
Post a Comment